In English: Life across the cell membrane
A cell’s surface membrane is its ultimate frontier. It seals the cell, separating the ordered metabolic processes that sustain life from the chaotic external environment. Essential though this barrier may be, no cell can survive without the exchange of raw materials, energy and information with its environment, and so the membrane also has to serve as a relay station where this bustling transfer of material and signals from the outside world happens.
We study membrane function in animal cells. Like all cell membranes, animal cell membranes possess the capacity to selectively pump or absorb small nutrients and minerals and swallow up or export lager cargoes life fats. They also sense chemical messages from their environment, which is very important for animal cells since these messages come from their neighbors, enabling all the cells that make up the animal to act in a co-ordinated way: these messages tell cells when to grow, when to move or even when to die for the good of the whole organism. Lacking a stiff cell wall, animal cell membranes must also interact with internal or external scaffolds to move along during development or healing, and allowing them to knit together into larger tissues.
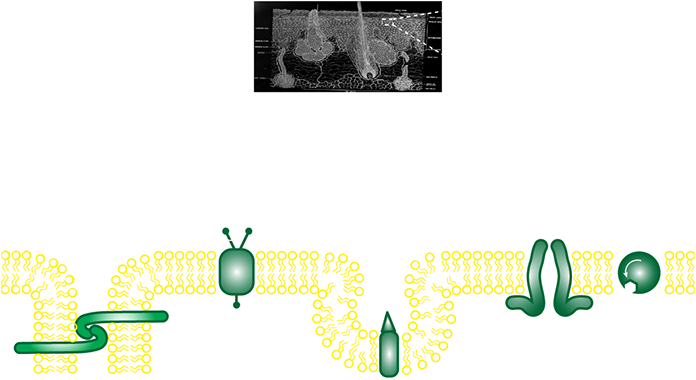
At just 1/1000 the thickness of the finest human hairs, the cell membrane is made of a layer of lipids just two molecules thick, arranged head-to-tail. Whilst these form a barrier to seal the cell, the membrane is studded with proteins that allow the cell to relay information and material with its surroundings. Tasks for the proteins include (from left to right) anchoring the cell to its surroundings and neighbors, relaying information from outside the cell, capturing and “swallowing” large cargoes like fat droplets (LDL), making pores to allow uptake of minerals, and pumping small nutrients like sugars.
First two images courtesy of Wikimedia commons.
Whilst the cell membrane itself is a vanishingly thin layer of lipids just two molecules thick, we tend to associate all of these membrane functions with the many thousands of genes that produce specific proteins, which either attach to or embed in the membrane; from here, these membrane proteins relay signals and materials across the membrane, or anchor it to stiff scaffolds. However, in nucleated cells like those found in humans, a special family of lipid molecules - the inositol lipids - control many of these membrane proteins, sticking them onto the membrane or switching them on or off after they arrive. We suspect that the lipids’s overarching role is actually to perform the crucial function of synchronizing all the many bustling material and information exchanges occurring at the cell membrane, by keeping all of these membrane proteins in the cell organized and coordinated.
Finding out exactly how the lipids achieve this is a crucial topic for research, since failure of cell membranes to carry out their varied tasks harmoniously is associated with many diseases, from cancer to the common cold. Indeed, drugs that prevent cells from making certain types of inositol lipids are proving to be potent inhibitors of the aberrant growth signals that drive some cancer cells. Nevertheless, we still possess a very incomplete understanding of how inositol lipids are produced in the appropriate amounts and places in cell membranes, enabling them to precisely control the ceaseless flow of different materials and signals, as well as the constant assembly and reorganization of cellular scaffolds. Only armed with a more precise understanding of how this specificity is achieved on cell membranes will we be able to develop drugs and other treatments that are specifically tailored to the individual processes that go wrong in disease. Attaining this basic knowledge is the central mission of our lab.
With Jargon: Membrane Homeostasis in Health and Disease
Uniquely among the phospholipids, phosphatidylinositol can be phosphorylated at any of three hydroxyl groups on its inositol head that are exposed to the cytoplasm. Any combination can and does occur in animal cells, creating a family of seven regulatory lipids. The 3D representation of lipid metabolism is taken from McCartney AJ, Zhang Y, Weisman LS. Phosphatidylinositol 3,5-bisphosphate: Low abundance, high significance. Bioessays. 2013 Oct 28;36(1):52–64.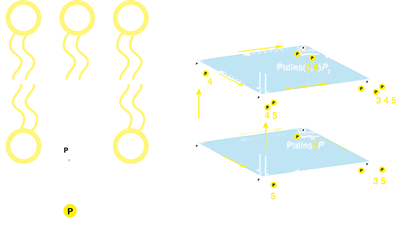 Healthy cellular function demands the co-ordination of assorted signals, molecular traffic and cytoskeletal attachment at membranes. Although protein function is usually the focus of research into these processes, inositol-containing phospholipids (see left) are absolutely crucial to membrane function in eukaryotes. They act as substrates in signaling reactions, recruit adaptors for membrane traffic, activate components of the cytoskeleton, as well as many other functions including the control of ion flux. How are these lipids and their protein ligands normally organized and co-ordinated? What homeostatic mechanisms maintain a stable lipid and protein composition in the face of membrane turnover?
Healthy cellular function demands the co-ordination of assorted signals, molecular traffic and cytoskeletal attachment at membranes. Although protein function is usually the focus of research into these processes, inositol-containing phospholipids (see left) are absolutely crucial to membrane function in eukaryotes. They act as substrates in signaling reactions, recruit adaptors for membrane traffic, activate components of the cytoskeleton, as well as many other functions including the control of ion flux. How are these lipids and their protein ligands normally organized and co-ordinated? What homeostatic mechanisms maintain a stable lipid and protein composition in the face of membrane turnover?
Answering these basic questions is crucial, because genetic diseases ranging from cancer to hereditary hearing loss are caused by disruption of membrane function resulting from mutations in inositol lipid metabolizing enzymes. Furthermore, many bacterial and viral pathogens re-model host cell membranes by actively disrupting inositol lipid distribution .
The overall aim of the lab is therefore to delineate the mechanisms of membrane organization and homeostasis, and how these mechanisms are altered in genetic and infectious disease. We use an array of state of the art methods, including live cell imaging, single molecule, super-resolution and chemical genetic approaches, supported by conventional molecular/cellular techniques, to probe the molecular scale organization of membranes. We interrogate specific protein-lipid complexes in both healthy cells and infectious or hereditary disease models. We are fully equipped with the necessary expertise and instrumentation, which combined with links to an established and growing network of collaborators in Pittsburgh and beyond, enables us to tackle these tough but crucial research questions. The lab thus represents an excellent environment in which to master many of these cutting edge techniques whilst tackling fundamental but health-relevant research questions.
More technical details of specific research projects that we are embarked on can be found in the “projects” page.
Department of Cell Biology