1) Nanoscopic organization of the plasma membrane by inositol lipids
A super-resolution STORM image of a probe for the plasma membrane-resident lipid PtdIns(4,5)P2. Image shows the lipid distribution with approx. 40 nm resolution.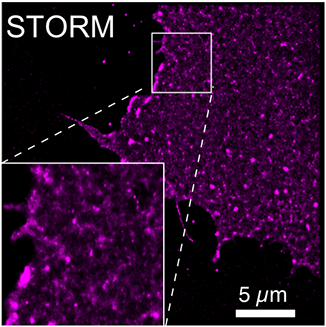 The inositol lipids interact with and regulate all sorts of protein complexes on the plasma membrane. Prominent examples include nascent endocytic compartments and focal adhesions, growth factor receptors, small G-proteins and even ion channels. Inositol lipids such as PtdIns(4,5)P2 or PtdIns(3,4,5)P3 can either recruit or directly activate these complexes - but the mechanistic details of how this happens in space and time are still hazy; are the complexes regulated by acute changes in the local concentrations of the lipids, triggering their assembly and/or activation? Or do local “hot spots” of concentrated lipids acts as pre-defined hubs where recruitment/activation occurs? Conversely, do the lipids even serve as localization factors at all, or do protein-protein interactions amongst the complex components instead concentrate the lipids for the lifetime of the complex?
The inositol lipids interact with and regulate all sorts of protein complexes on the plasma membrane. Prominent examples include nascent endocytic compartments and focal adhesions, growth factor receptors, small G-proteins and even ion channels. Inositol lipids such as PtdIns(4,5)P2 or PtdIns(3,4,5)P3 can either recruit or directly activate these complexes - but the mechanistic details of how this happens in space and time are still hazy; are the complexes regulated by acute changes in the local concentrations of the lipids, triggering their assembly and/or activation? Or do local “hot spots” of concentrated lipids acts as pre-defined hubs where recruitment/activation occurs? Conversely, do the lipids even serve as localization factors at all, or do protein-protein interactions amongst the complex components instead concentrate the lipids for the lifetime of the complex?
Answering these questions is central to determining how specificity emerges from protein-lipid interactions, and consequently to understand how specific interactions fail in disease - and how therapeutic interventions might one day be tailored to specifically target aberrant interactions. A prominent example is the growth factor signaling pathways that are up-regulated in most cancers, which trigger changes to membrane organization and polarity necessary for both constitutive growth and survival, as well the loss of polarity and a more motile phenotype associated with the epithelial to mesenchymal transition. Selective disruption of these inositol lipid-dependent processes in cancer cells must be balanced against maintaining the essential housekeeping transport and signaling functions occurring in the neighboring healthy tissue – and can only be achieved by understanding how specificity is achieved in building the protein-lipid complexes that mediate both disease-associated and healthy cellular function.
Tackling these questions requires approaches than can (i) resolve individual molecular complexes within the crowded molecular milieu of the plasma membrane, (ii) localize the lipids themselves in the plane of the membrane, and (iii) track both sets of components’ dynamic interaction in real time. For this we primarily turn to advanced optical imaging-based single molecule approaches. The lab is equipped with a Nikon TIRF microscope with latest generation sCMOS camera for multi-color single molecule imaging for dynamic real-time measurements of protein complexes and fluorescent lipids and lipid reporters. An A1R dual resonant/galvo driven confocal scan head allows supporting measurement of molecular population dynamics through fluorescence bleaching and activation experiments. Through close collaboration with the Center for Biologic Imaging, we also have access to super-resolution imaging techniques (such as STORM) for high resolution, static imaging of membrane organization.
2) Homeostatic control of inositol lipid signaling in asthma and vascular hypertension
Vascular hypertension and asthma are very different diseases, yet both share a common etiology: in each, over-contraction of the vascular or airwayCa2+ signaling in smooth muscle. (1) contractile hormones and inflammatory mediators activate G-protein coupled receptors at the cell surface, activating PLC inside cells to in turn (2) trigger Ca2+-release from internal SR stores to stimulate constriction of airways or blood vessels. (3) Ca2+ is continually removed from the cell by pumps and exchangers at the cell surface, so (4) Ca2+ influx is required to sustain constriction. Note, all these steps require the lipid PIP2. The action of medicines that relieve constriction in asthma and hypertension are indicated. These include ACE inhibitors, Angiotensin Receptor Blockers (ARB), Calcium Channel Blocks (CCB), anti-cholinergics and Inhaled Corticosteroids (ICS).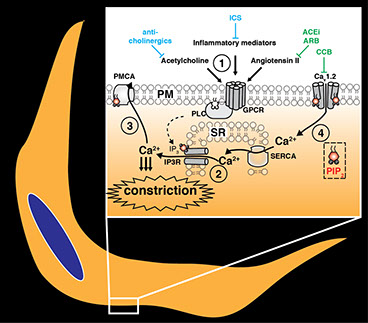 smooth muscle cells is central to pathogenesis. In airway and vascular smooth muscle, the PI(4,5)P2-coupled phospholipase C signaling pathway is central to transduce tonic or inflammatory stimuli into smooth muscle contraction. Many existing bronchodilating or vaso-relaxing therapies antagonize the various inputs into this central pathway, and these are often used in combination to attain greater therapeutic efficacy. However, to date there is little known about how modulating the convergent lipid signaling pathway itself might be used for therapeutic benefit. Our lab is investigating this topic at a fundamental cell biological level.
smooth muscle cells is central to pathogenesis. In airway and vascular smooth muscle, the PI(4,5)P2-coupled phospholipase C signaling pathway is central to transduce tonic or inflammatory stimuli into smooth muscle contraction. Many existing bronchodilating or vaso-relaxing therapies antagonize the various inputs into this central pathway, and these are often used in combination to attain greater therapeutic efficacy. However, to date there is little known about how modulating the convergent lipid signaling pathway itself might be used for therapeutic benefit. Our lab is investigating this topic at a fundamental cell biological level.
PI(4,5)P2 is a lipid specifically enriched in the cytoplasmic leaflet of cell membranes that regulates scores of proteins, thus controlling cell structure, nutrient important and export, as well as signal transduction. The phospholipase C pathway triggers PI(4,5)P2 breakdown to produce second messenger molecules that stimulate Ca2+-activated contraction. However, to sustain this signaling, and also to sustain the many other essential cellular functions associated with PI(4,5)P2, the cell must automatically adjust re-synthesis of the lipid to maintain a constant supply. This is a logistically demanding cellular exercise, employing up-regulation of several enzymes necessary for synthesis, as well as specialized transport machinery that rapidly re-cycles lipid breakdown products back to the ER for their re-assembly and their return to the plasma membrane. Despite the established importance of this complex cellular machinery, we still know almost nothing about how it is regulated. Our lab employs high-resolution imaging approaches to study the molecular dynamics employed by these signaling events in real-time at the single cell level. We are determining the key regulatory steps involved in PI(4,5)P2 re-synthesis, and how this modulates Ca2+-signaling output. We hope that this knowledge will lead to new therapeutic strategies to reduce pathological smooth muscle cell contraction in vascular hypertension and asthma.
3) Lipid contributions to membrane identity in health and disease
The complex internal organization of the cell into distinct membrane bound organelles creates a fundamental problem for the membrane proteins that traffic through it: how to target specific membranes, and restrict their activity solely to these membranes? The inositol lipids are found exclusively in the cytosolic leaflets of organelle membranes linked by the secretory and endocytic pathways; their interconversion corresponds loosely with the flow of membranes between compartments. A popular model is therefore that inositol lipids serve principally to restrict suites of proteins to their appropriate membrane compartment. Put another way, the presence of a given inositol lipid in a particular membrane contributes to that membrane’s unique identity, and helps the appropriate proteins identify it. As membrane flows through the endocytic pathway, inositol lipid modifying enzymes alter the lipid head groups to correspond with their new cellular locale.
This model has important ramifications for a host of rare monogenic syndromes caused by mutations that inactivate inositol lipid phosphatases. These diseases include Lowe and Joubert syndromes (caused by mutations in PtdIns(4,5)P2 5-phosphatases) and Charcot-Marie-Tooth disease (myotubularin PtdIns3P/PtdIns(3,5)P2 3-phosphatases). These diseases affect a variety of organs (such as eyes, kidney and brain) but appear to be driven by failures in the membrane trafficking systems of cells in the affected tissues. A reasonable hypothesis is therefore that the cellular defects that cause these diseases emerge from accumulation of inositol lipids in inappropriate membrane compartments, leading to the accumulation of the wrong suites of effector proteins and the eventual failure of specific traffic pathways in the cell. In short, there is a breakdown of membrane identity in these cells.
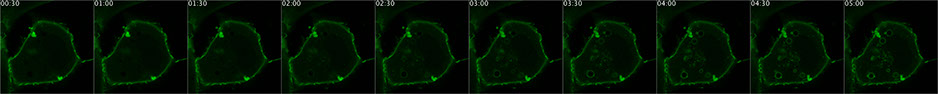 A fluorescent PtdIns(4,5)P2 biosensor detects an experimentally induced accumulation of the lipid on endosomes in a living cell
A fluorescent PtdIns(4,5)P2 biosensor detects an experimentally induced accumulation of the lipid on endosomes in a living cell
We aim to test this hypothesis, taking advantage of our lab’s expertise both in probes to localize inositol lipids in living cells, and in chemical genetic approaches to manipulate the lipids in specific membrane compartments. The project entails both attempts to correct cellular deficits in cellular models of the diseases, as well as testing the key hypotheses by re-creating phenotypes in otherwise healthy cells. The approach relies heavily on molecular genetics, chemical genetics, genome editing and live-cell imaging.
Department of Cell Biology