Emerging role of deubiuqitinase family members in tumor initiation, progression and invasion attracts critical attention in cancer field. To systematically search for deubiquitinase whose misexpression predisposes mammary gland epithelial cells to become cancerous or enhances breast tumor invasion, we have conducted a high throughput screening of approximately 100 deubiquitinase library (Fig. 7) . Our efforts led to the identification of USP11 as a potent oncogene with its elevated expression directly triggering malignant transformation of mammary gland epithelial cells. We further observe that USP11-driven mammary oncogenesis is due to overriding XIAP that results in antagonizing apoptosis. We are now addressing the mechanism by which XIAP is regulated by coordination between E3 ligase (XIAP acts as self-ubiquitin protein ligase) and deubiquitinase (USP11). In addition, we are determining how failure in the proteolytic regulation of XIAP by USP11 would affect cellular apoptotic feature that in turn triggers tumorigenesis by utilizing a breast cancer transgenic mouse model.
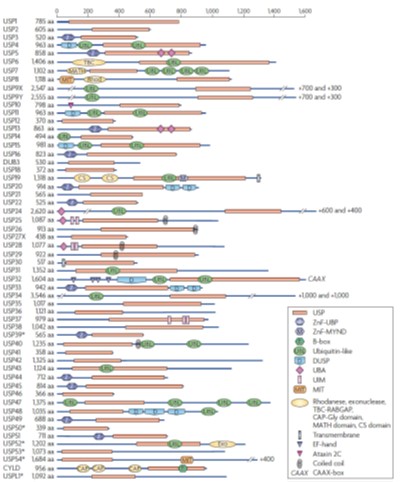
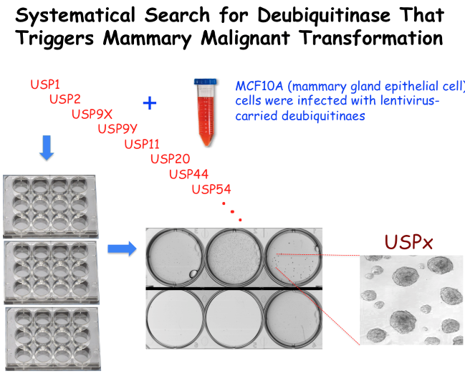
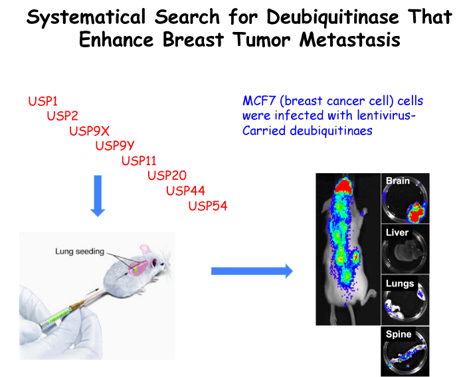
Figure 7. A strategy to identify critical deubiquitinases promoting tumor initiation or invasion. (A) Diagram of ~100 deubiquitinase family members. (B) A strategy to identify critical deubiquitinases promoting tumor initiation. (C) A strategy to identify potent deubiquitinases promoting tumor invasion.